No products
Western Blot
The following protocol is an outline of a traditio nal Western blotting protoc ol for the detection and characterization of a particular protein or bio-molecu le in a sample. Protein sample mixtures are first resolved by size using sodium dodecyl sulfate po lyacrylamide gel electrophoresis (SDS-PAGE). Then electro-transfer, or blotting of samples on the gel to a nitrocellulose or polyvinylidene fluoride (PVDF) membrane is performed. Specific proteins are then detected on the blot by a series of blocking, incubating and washing steps with the appropriate buffers and antibody probes. Wester n blots resolve complex populations of proteins and provide data on the molecular weight and abundance of a particular protein of interest in a sample mixture.
Important NOTES:
- A single-page quick protocol is included at th e end of this document. Print this page for quick bench-top reference.
- Protein sample preparation methods vary widely in order to deliver protein in the conformation required for a particular assay. In this protocol, denaturing and reducing conditions are used as they are most common ly applied to Western blotting procedures and typically provide for the highest sensitivity and best resolution. However, users can make changes to the sample preparation and PAGE procedures in order to run non-denaturing or non-reducing conditions. Non-reducing conditio ns require no change to the protocol apart from the exclusion of reducing reagents from preparative buffers. Non-denaturing conditions require a more dramatic change in the PAGE protocol, and is not elucidated here.
- Western blot probes vary widely. Antibodi es can be selected with a wide range of specificities and reactivities to a protein of in terest. This protocol details the generic methodology utilizing a primary antibody for protein recognition followed by a secondary antibody. The secondary antibody, which is against the primary, and conjugated to a chemiluminescence signal amplification enzyme, Horseradish Peroxidase (HRP) for visualization. Alternative probes may have othe r blotting requirements not included in this protocol. If so, consult with the manufacturer ’s guidelines of the reagent in question for further instructions.
Procedures
- 1.Prepare 2x LDS Buffer with 2x DTT:
Reagent Volume per Sample (μL) Sample Number Preparative Volume (μL) 4x LDS Sample Buffe 10 x _____ = 1M DTT 4 x _____ = Deionized Water 6 x _____ = - 2.Prepare protein samples for loading. Add 15uL of protein sample (diluted in deionized water if necessary) to 15uL of 2x LDS buffer containing 2x DTT.
- 3. Boil samples at 100 o C for 5 minutes.
- 4. Set up the PAGE gel. Quickly rinse the gel with deionized water, remove the comb and rinse the wells with running buffer, place it in the electrophoresis chamber, fill the upper trough with 1x running buffer.
- 5. Load 15uL of each prepared sample into a well on the gel in an established order.
- 6. Run the gel at a constant 180V for approximate ly 45-60 minutes or watch the position loading dye to stop the run.
- 7. Setup blotting sandwich and apparatus. Crack open the gel casing to access the gel. Cut away the wells and thick bottom portion of the gel. In a tray filled with 1x transfer buffer, assemble the blotting sandwich in the following order: (bottom) - filter-paper – gel – nitrocellulose - filter- paper - (top) . Place the blotting sandwich on the electroblotting apparatus as follows:
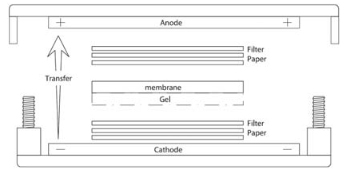
- 8. Block the membrane in 5% milk, 1x TBST fo r 30 minutes to 1hr on a rotating shaker.
- 9. While blocking, dilute the primary antibody in 5% milk, 1x TBST. Prepare enough diluted antibody to cover the blot during shaking incubation.
- 10. Decant blocking solution, rinse the membrane with 1x TBST, wash the membrane 2 – 3 times with 1x TBST buffer, each time 5 – 8 minutes with shaking on a rotating shaker.
- 11. Incubate the membrane with the diluted primary antibody for 30 minutes to 1hr on a rotating shaker.
- 12. If no secondary antibody or additional probes are necessary, proceed directly to step 10. Otherwise, discard the diluted primary antibody an d wash with 5% milk, 1x TBST for 5 – 8 mins on a rotating shaker. Repeat this wash step 2 additional times for a total of 3 washes.
- 13. While washing, dilute the secondary antibody in 5% milk, 1x TBST. Prepare enough diluted antibody to cover the blot during shaking incubation.
- 14. After washing, incubate the membrane with the diluted secondary antibody. Incubate for 30 minutes to 1hr on a rotating shaker.
- 15. Discard the diluted primary/secondary antibody and wash with 1x TBST for 3 times, each time 5 - 8 minutes on a rotating shaker.
- 16. Proceed with the appropriate ECL substrate incubation and chemiluminescence/fluorescence detection as specified by the reagents manufacturer.
Reagents & Buffer
50mL 20x NuPAGE MOPS SDS Running Buffer
950mL Deionized Water
50mL 20x NuPAGE Transfer Buffer
200mL 99-100% Methanol
750mL Deionized Water
10mL 10% Tween-20
890mL Deionized Water
100mL 10x Tris-Buffered Saline (500mM Tris pH 7.4, 1.5M NaCl)
10mL 10% Tween-20
50g Blotting Grade Non-fat Dry Milk
890mL Deionized Water (Bring final volume to 1L)
| Disposables/Reagents |
| PAGE Gel, 4-12%, or 4 -20 % Bis-Tris |
| SDS Sample Buffer (4x) |
| Western Blot Protein Standard |
| Dithiothreitol (DTT) |
| Beta-mercaptoethanol |
| MOPS or MES SDS Running Buffer (20x) |
| NuPAGE Transfer Buffer (20x) |
| Laboratory Grade Methanol |
| Blotting Sandwiches |
| Tris-Buffered Saline (10x) |
| Tween-20 |
| Blotting Grade Non-fat Dry Milk |
| TBS Buffer |
| SuperSignal West Dura Extended Duration Substrate |
| Equipment |
| Gel box |
| Power Supply (100-120/220-240V) |
| Semi-dry Electroblotting System |
| Chemidoc Image System or X-ray film |
