Cart
0
Product
Products
(empty)
No products
To be determined
Shipping
$0.00
Total
Product successfully added to your shopping cart
Quantity
Total
There are 0 items in your cart.
There is 1 item in your cart.
Total products
Total shipping
To be determined
Total
Dot Blot
The following protocol is a simplified alternative method , the Dot Blot, to traditional Western blotting for the detection of the presence or absence of a particular protein or bio-molecule in samples. Dot Blot differs from Westerns in that proteins in the samples are not resolved by size prior to blotting. This method is better suited for comparin g relative abundance of a target protein in a number of samples in parallel ( low-, medium-, high-throughput ).
Procedures
- 1.Label the nitrocellulose blotting membrane for th e ease to locate your individual sample after blotting. For example, for 96-well sample dot bl ots, designate one corner of the membrane as well number A1, another corner as H12. Use indelible marker that will not bleed during processing.
- 2.Place the labeled membrane on a filter paper. Se cure the edges of the membrane with tape or a weight to prevent the edge curling.
- 3.Pipette 0.5-2.0uL of each sample onto separate pre-determined locations on the blot. A multi- channel pipette/96 or 384-pin head may be used to spot multiple samples at once. The samples should be absorbed to the membrane immediately without any beading on the surface.
- 4.Block the membrane in 5% milk, 1x TBST fo r 30 minutes to 1hr on a rotating shaker.
- 5.While blocking, dilute the primary antibody in 5% milk, 1x TBST. Prepare enough diluted antibody to cover the blot during shaking incubation.
- 6.Decant blocking solution, rinse the membrane with 1x TBST, wash the membrane 2 – 3 times with 1x TBST buffer, each time 5 – 8 minutes with shaking on a rotating shaker.
- 7.Incubate the membrane with the diluted primary antibody for 30 minutes to 1hr on a rotating shaker.
- 8.If no secondary antibody or additional probes are necessary, proceed directly to step 10. Otherwise, discard the diluted primary antibody an d wash with 5% milk, 1x TBST for 5 – 8 mins on a rotating shaker. Repeat this wash step 2 additional times for a total of 3 washes.
- 9.While washing, dilute the secondary antibody in 5% milk, 1x TBST. Prepare enough diluted antibody to cover the blot during shaking incubation.
- 10.After washing, incubate the membrane with the diluted secondary antibody. Incubate for 30 minutes to 1hr on a rotating shaker.
- 11.Discard the diluted primary/secondary antibody and wash with 1x TBST for 3 times, each time 5 - 8 minutes on a rotating shaker.
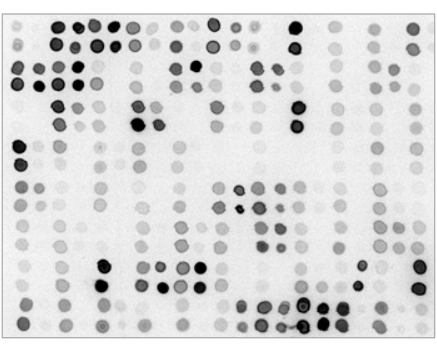
- 12. Proceed with the appropriate ECL substrate incubation and chemiluminescence/fluorescence detection as specified by the reagents manufacturer. A sample Dot-Blot (spotted by 384- pin head ), with 0.5 ul/spot:
Reagents & Buffer
1x TBST Buffer (1L
100mL 10x Tris-Buffered Saline (500mM Tris pH 7.4, 1.5M NaCl)
10mL 10% Tween 20
890mL Deionized Water
1x TBST Buffer + 5% Milk (1L) Note: Powdered non-fat dry milk must be thoroughly dissolved into buffer before Tween-20 addition so that no sediment remains.
100mL 10x Tris-Buffered Saline (500mM Tris pH 7.4, 1.5M NaCl)
10mL 10% Tween 20
50g Blotting Grade Non-fat Dry Milk
890mL Deionized Water
