No products
Recombinant protein expression and purification (NB-SC101)
EXPECT THE FASTEST TURNAROUND TIME WHEN WORKING WITH US
Please fill out the protein production request form here and send to us for protein production service.
Novatein Biosciences offers recombinant protein production services to accelerate your research and development, by using highly efficient recombinant protein expression strategies. A general outline of our protein production service is as follows:
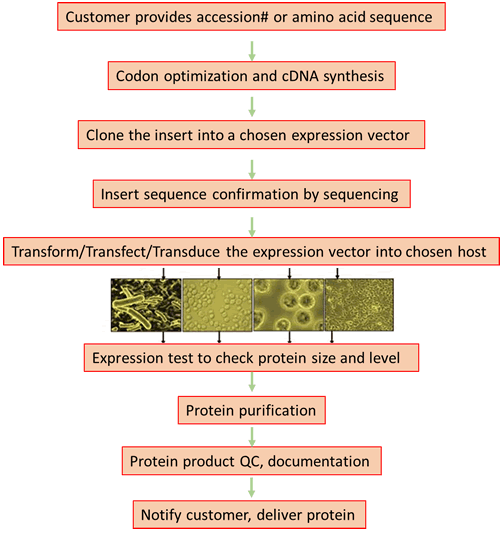
- Codon optimization and cDNA synthesis: Customer provides the accession number or the amino acid sequence of the target gene. We will do free codon optimization and cDNA synthesis.
- Gene cloning: The synthesized cDNA will be cloned into an expression vector ( for host of any of the bacterial/ yeast/ baculoviral or mammalian ) and the sequence is authenticated by DNA sequencing.
- Gene expression: the target gene will be expressed in an optimized conditions. Expression level and integrity will be checked by SDS-PAGE and/or Western blot.
- Protein Purification: The protein will be purified by column chromatography procedures (affinity and gel filtration).
- Protein Identification: The purified protein will be separated on SDS-PAGE gel and stained/ transferred to membrane for immunoblot. Mass spectrometry can be performed for an additional charge.
Note: Customer can also send us their expression vector to start protein production. Or send us protein preps for further purification and characterization.
Novatein Biosciences has extensive experience in the recombinant protein expression and purification. Express your proteins with or without tags in
- Bacterial cells: various strains (4-6 weeks)
- Insect cells : S2, Sf9, Sf21, and Hi5 cells (8-10 weeks)
- Mammalian cells: CHO, HEK293 and others (5 – 7 weeks)
With our over a decade years of experience, we are experts in creating and optimizing stable and transient expression for secreted or intracellular, cytosolic or periplasmic, tagged or untagged proteins. We assess expression levels using the SDS-PAGE, Western, ELISA or functional analysis. We optimize protein expression by modification of, promoter, ribosome binding site, vector copy number, host cell, growth condition including media and additives, time, and temperature
In addition, we offer the following services:
- Inclusion body purification
- Refolding with typical or preferred protocols
For protein purification, we have the capability to run the following:
Affinity Chromatography
Ni- NTA, Talon resins, Glutathione Sepharose, Streptavidin Sepharose, and anti-Flag
Ion Exchange Chromatography
Mono-Q, Q Sepharose Fast Flow, Resource Q, Q Sepharose XL, ANX, DEAE Sepharose, SP Sepharose, CM Sepharose
Hydrophobic interaction Chromatography
Phenyl and (CH2)n Sepharose
Immuno-Affinity Chromatography
Protein A and Protein G Sepharose
Size Exclusion Chromatography
Antibody and Fc Fusion Protein Production and Purification Services (low or very low endotoxin levels)
Novatein Biosciences has extensive experience in the expression and purification of recombinant antibody and Fc fusion proteins from microgram to kilogram scales in CHO and HEK 293 cells. Using our proprietary fully optimized secretion signal and linker sequences, we can efficiently complete a project from gene construction to Fc fusion protein purification. Fc fusion can be N-terminal or C-terminal. Multiple Fc backbones are available to choose from.
Fast turnaround:
1-2 weeks for codon optimization and synthesizing the gene of interest.
1 week for molecular construction of recombinant Fc fusion proteins.
Protein A is used for recombinant antibody purification. Please request if multiple purification methods are required.
Recombinant Protein Production Using the Insect Cell Expression System
- Gene of interest (GOI) is cloned into a suitable donor plasmid containing a mini-Tn7 element.
- The recombinant donor plasmid is then transformed into E. coli cells which contain a bacmid with a mini-attTn7 target site and a helper plasmid. The mini-Tn7 element on the donor plasmid facilitates transposition of the GOI into the target site on the bacmid resulting in insertion of the GOI into the bacmidDNA.
- Recombinant bacmid DNA is extracted and purified (for efficient homologous recombination, pure DNA is required).
- Insect cells are transfected with the recombinant bacmid DNA to produce recombinant baculovirus particles with GOI (P1 virus generation)
- The insect cell cultures are infected with the purified P1 stock to create a P2 stock (100 ml serum free medium).
- A high quality high titer working virus stock P3 (~500 ml) is produced by infecting Sf9 cells with a P2 virus at a low multiplicity of infection (MOI=0.1)
Optimization of heterologous protein expression and purification of protein by successive chromatography techniques
